From requirements back to the source
The purification technology required to produce the appropriate water quality for the electrolyzer is also determined by the feed water source. The use of drinking water and groundwater for industrial production processes is becoming increasingly limited, meaning that surface water is considered the most sustainable source for industry.
Please contact us.
Required water quality
The use of surface water or, for example, purified wastewater as a source affects the total operating costs of the installation. Purified wastewater often has a high salt content and contains relatively many suspended and organic substances. This increases the number of treatment steps and therefore the operating costs, including energy consumption. When using waste water, the discharge of the concentrate must also be taken into account, which is usually not suitable for direct discharge into surface water.
To produce the required water quality, a combination of techniques is usually chosen:
- Ultrafiltration for separating suspended solids
- Softening to remove or bind calcium
- Reverse osmosis for desalination and separation of organic substances
- Ion exchange and/or electro-deionization to remove residual salts
The quality of the demineralized water from the purification plant normally meets the requirements for the electrolyzer. However, storage and transport of the demineralized water from the purification plant to the hydrogen factory can change the quality. This is due to the absorption of gases such as oxygen and carbon dioxide or metal particles from the pipe system. To ensure continuous high water quality for the electrolyzers, a post-purification or “point-of-use” system is installed at the inlet of the electrolyzer. This system usually consists of a combination of degassing and electrodeionization (EDI) or ion exchange.
Certain water quality requirements also apply to cooling water to limit pollution and corrosion in the cooling tower. Furthermore, the water quality also determines the blowdown volume of the cooling tower. The dirtier the water, the more drain water and also the more waste water. This creates a larger water footprint and a less efficient process.
Lime deposits (CaCO3) in the cooling system can be prevented by means of softening or chemistry. During the softening process, calcium is captured by an ion exchange resin and exchanged for sodium. Sodium carbonate (Na2CO3) has a higher solubility in water and is less likely to precipitate on the heat exchangers in the cooling system. Chemical or anti-scalant can be considered as an alternative, depending on the water hardness and water flow rate.
By (partial) desalination of the feed water for the cooling tower, the use of chemicals and the blowdown volume of the cooling tower can be kept to a minimum. However, not every cooling tower is suitable for this water quality, which requires customization. Want to know more about low-chemical cooling? Then read our flyer.
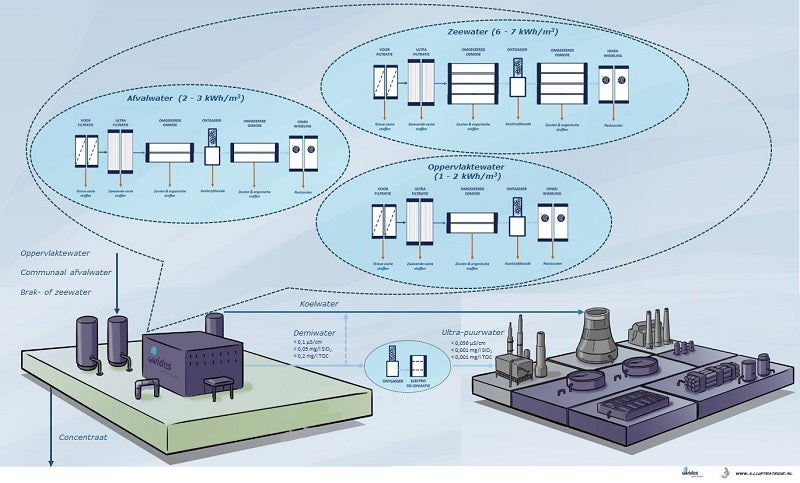
By choosing the right combination of techniques, in principle any water source can be used. But when choosing the water source, other interests must also be taken into account, such as:
- Availability of the water source, during the year and for the long term;
- Socially responsible aspects such as
* CO2 footprint per source
* competition with drinking water supplies or nature